What is an Atom?
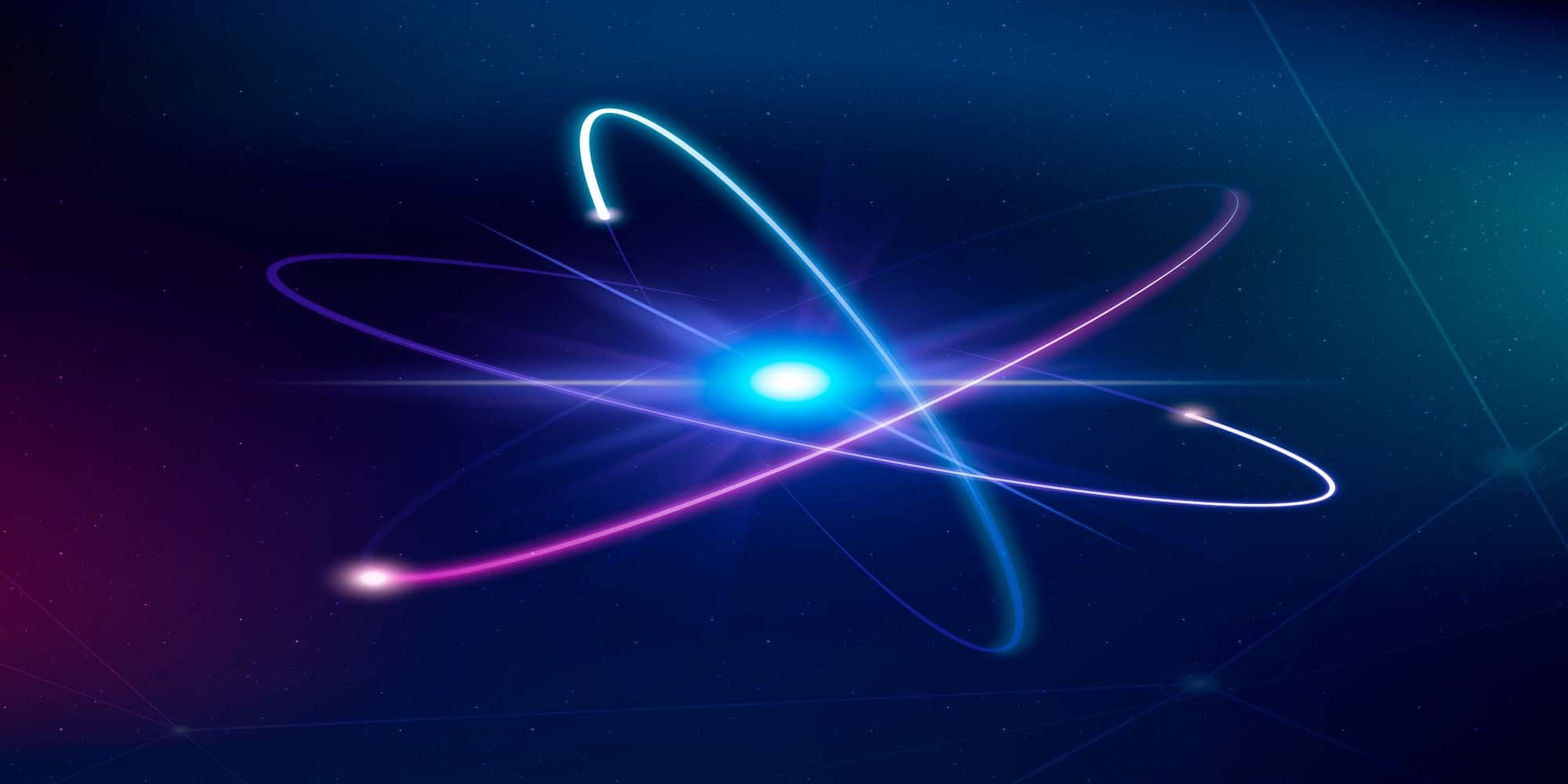
So, what is an atom?
In simple words, an atom is a particle that consists of a nucleus surrounded by electrons that occupy atomic orbitals.
Well, not so “simple”, for me at least!
If you are wondering why I am writing about it, it’s because I want to understand – the universe, the world, me. What’s it all about? What is my purpose?
As Socrates said, “the unexamined life is not worth living". So true!
The fundamental questions of our existence and purpose are extensively discussed in law, philosophy, and psychology. But I always keep finding a dead end when trying to find my answers (and I really try to find those answers).
Logic says (disclaimer: my logic, not common or any other type or form of logic), that to know why we are, we should know who we are, which means we should first discover what we are made of.
For now, I am focusing only on scientific facts (undeniably true statements accepted by the science community).
Of course, interpreted with my point of view (because I am a lawyer and I naturally tend to philosophize) and with a touch of humor (just because I am me).
So, considering that all things are made of atoms (all things – if you just pause for a second and realise what that means – wow!) I want to know.
Questions
-
What is an atom made of? I already know (using “know” in the widest possible meaning) that an atom is composed of a nucleus, with a cloud of one or more electrons surrounding it.
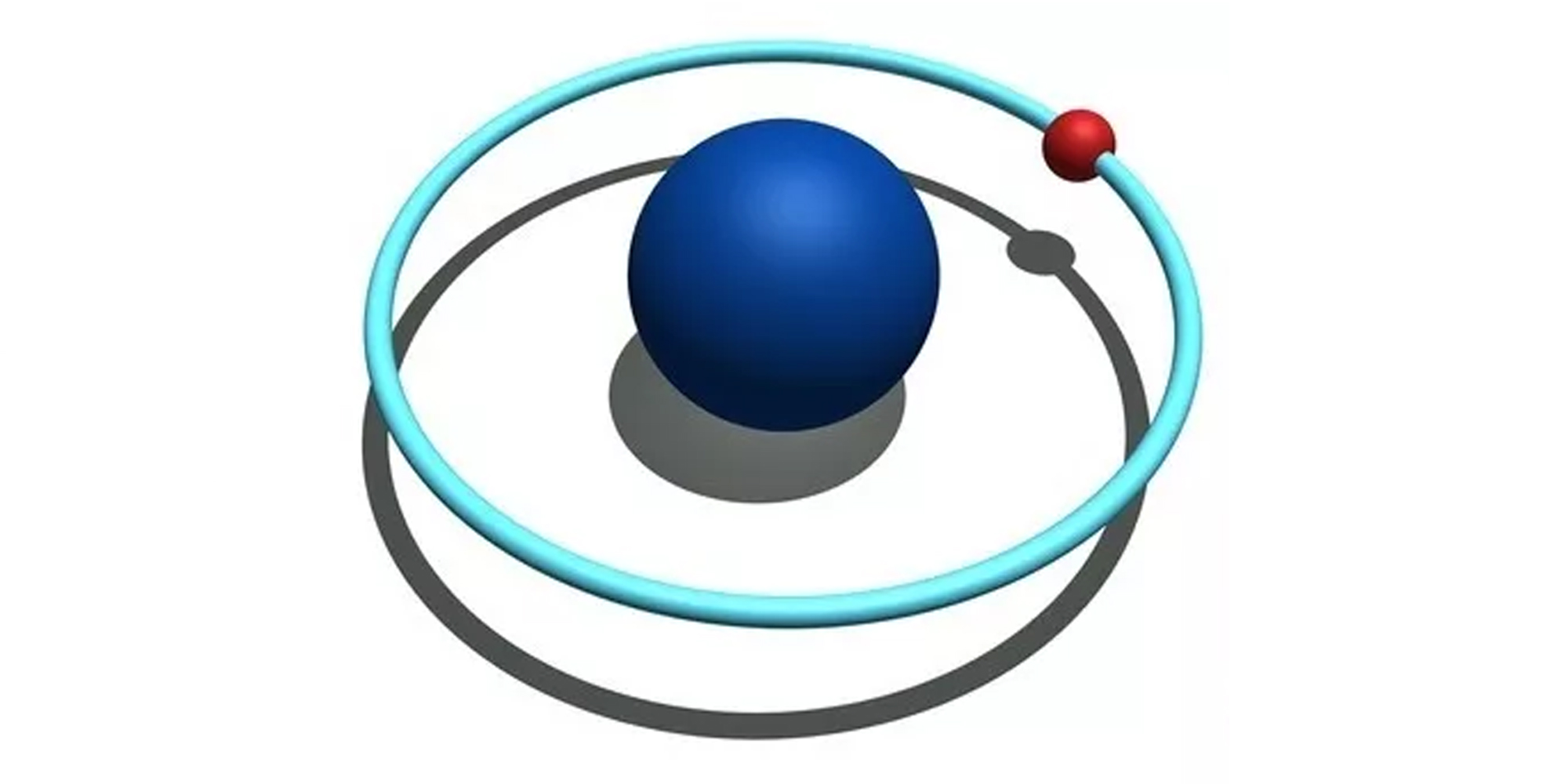
-
What is a nucleus? Nucleus is the small, dense, spherical center of an atom (the blue circle in the center of the figure above).
-
What is an electron? Electron is a subatomic particle that occupies atomic orbitals around the nucleus, where it's most likely to be found.
-
What is an electron made of? Electrons are elementary particles, meaning that scientists' current understanding is that they cannot be broken down into smaller components.
-
What is nucleus made of? A nucleus consists of protons and neutrons.
Does this ever end? Five million of the simplest atoms would fit across the head of a pin! One would think that the atom is the smallest particle in existence. But it’s not!
Atoms are further split into protons and neutrons and electrons. And then protons and neutrons are split even further.
-
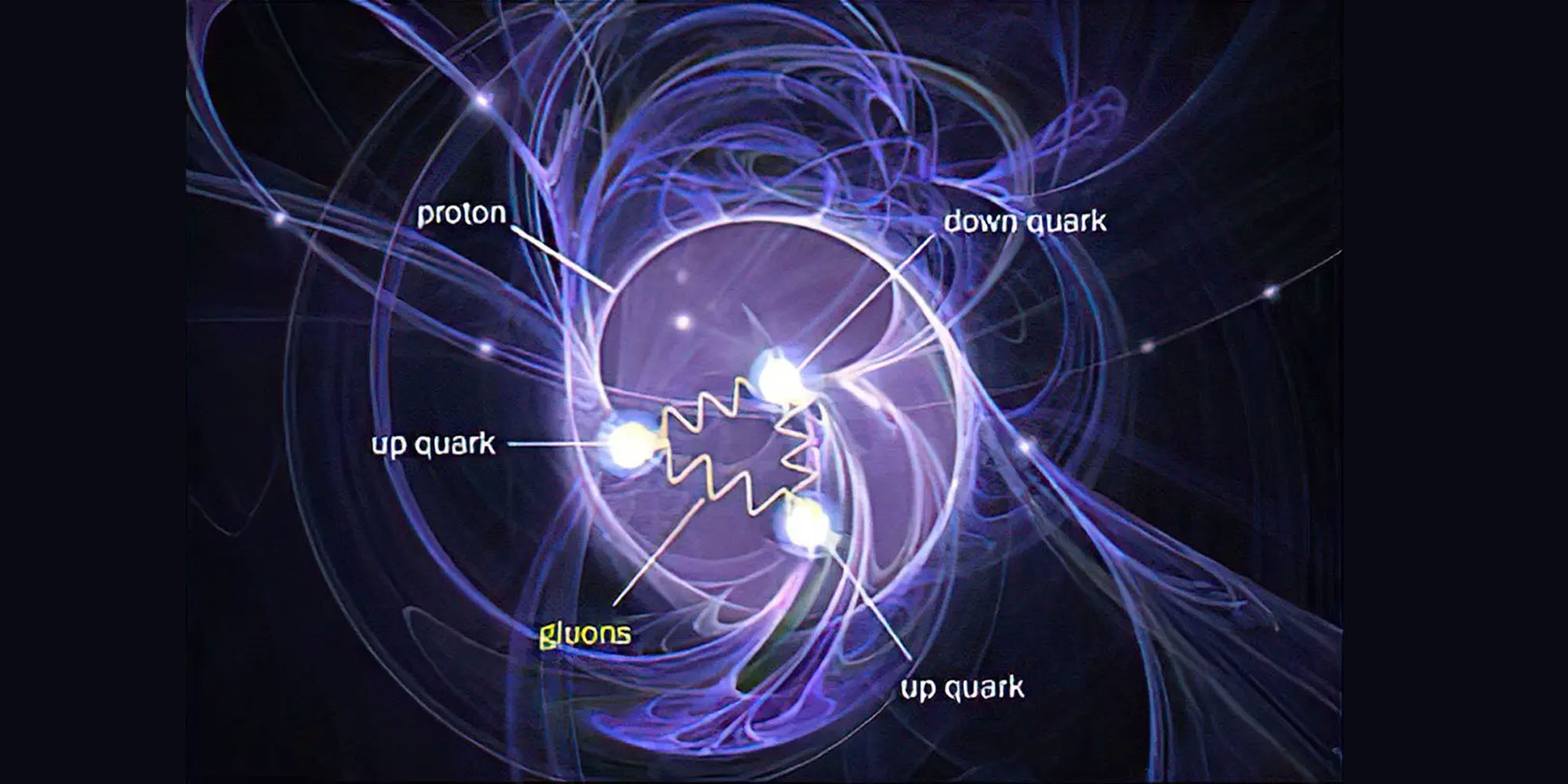
What are protons and neutrons made of? Protons and neutrons consist of quarks and gluons.

-
What is an atom, in a nutshell? An atom consists of a central nucleus (composed of protons and neutrons, which are further made up of quarks and gluons) surrounded by electrons that occupy atomic orbitals.
Conclusion
So, I finally reached the smallest particles – at least for now – until scientists split the quarks or electrons and discover even more particles.
Maybe with the right technology we could continue splitting particles indefinitely. Who knows?
For now, we can safely say that an atom is what everything is made of, and that all atoms are made of quarks, gluons and electrons. End of story.
But is it so?
Of course not! This definition does not even come close to explaining the magic that creates worlds.
I don’t even understand the basis – for example, what is really a quark?